Cardiology
Cardiac pathophysiology in Fabry disease
The information on this page is tailored for cardiologists. For more detailed information on the cause, inheritance, diagnosis and management of Fabry disease, please refer to our ‘About Fabry disease’ page.
There is evidence of cardiac involvement early in life, during adolescence in both sexes, with cardiac symptoms appearing roughly ten years earlier in males than females. Overall, around 90% of Fabry disease patients are affected by cardiomyopathy.1-3
Pathology in Fabry disease arises from accumulation of globotriaosylceramide (GL-3) which leads to cellular dysfunction. Specifically, a cascade of events is triggered which includes oxidative stress, compromised metabolism and cell death resulting in vascular dysfunction and tissue fibrosis.1,4 In relation to the cardiomyopathy seen in Fabry disease, research has shown that a high percentage of GL-3 is stored across a range of cardiac cells.5 It is thought this deposition leads to cell injury and a variety of tissue responses leading to progressive cardiac disease.
Click on the headings to learn about some of the proposed mechanisms of cardiac pathology.
GL-3 accumulation in cardiomyocytes impairs ventricle function as it impedes the ability of these cells to relax and stiffen as the heart pumps. It is likely these events are the cause of the diastolic dysfunction which is commonly seen in Fabry disease.
GL-3 accumulation also causes myofibrillolysis. This event has been associated with degradation of myofilamental proteins which impacts on the structure and function of the heart walls. It is suggested therefore, that cardiomyocyte impairment may in part be explained by proteolysis as a result of GL-3 deposition.
Cardiomyopathy of Fabry disease
Cardiomyopathy from Fabry disease is summarised in the diagram below.
Adapted from Pieroni et al 2021.6
Cardiac progression in Fabry disease
This graph summarises typical cardiac progression in Fabry disease patients.
Tap on each dot to learn more
Adapted from Eng et al 2007.7
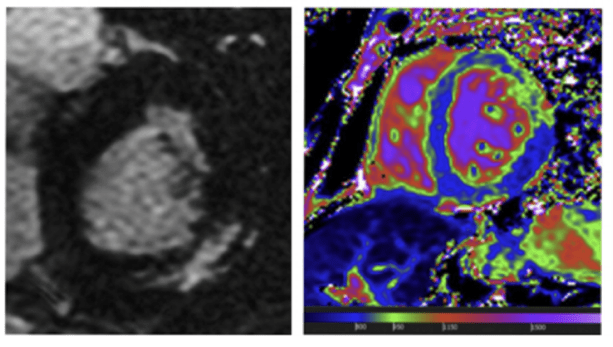
Reproduced with permission from Hagège A et al., 2019.8
Here, we see an MRI of a 65-year-old female patient with Fabry disease. You can see typical late gadolinium enhancement in the basal segment of the posterolateral wall (white myocardial area) and basal short-axis T1 mapping of the myocardium at 1.5 Tesla with homogenous low T1 values (in blue, corresponding to a T1 at 800 ms).
NP-NN-UKI-00031024
October 2024
- Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30.
- Linhart A, Kampmann C, Zamorano JL, et al. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007;28:1228–1235.
- Kampmann C, Perrin A, Beck M. Effectiveness of agalsidase alfa enzyme replacement in Fabry disease: cardiac outcomes after 10 years’ treatment. Orphanet J Rare Dis. 2015;10:125.
- Rombach SM, Twickler TB, Aerts JMFG, et al. Vasculopathy in patients with Fabry disease: current controversies and research directions. Molec Genet Metab. 2010;99:99–108.
- Hoffman B. Fabry disease: recent advances in pathology, diagnosis, treatment and monitoring. Orphanet J Rare Dis. 2009;4:21.
- Pieroni M, Moon JC, Arbustini EA, et al. Cardiac involvement in Fabry disease. JACC. 2021;77:922–936.
- Eng CM, Fletcher J, Wilcox WR, et al. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis. 2007;30:184-192.
- Hagège A, Réant P, Habib G, et al. Fabry disease in cardiology practice: literature review and expert point of view. Archives Cardio Dis. 2019;112:278–287.


